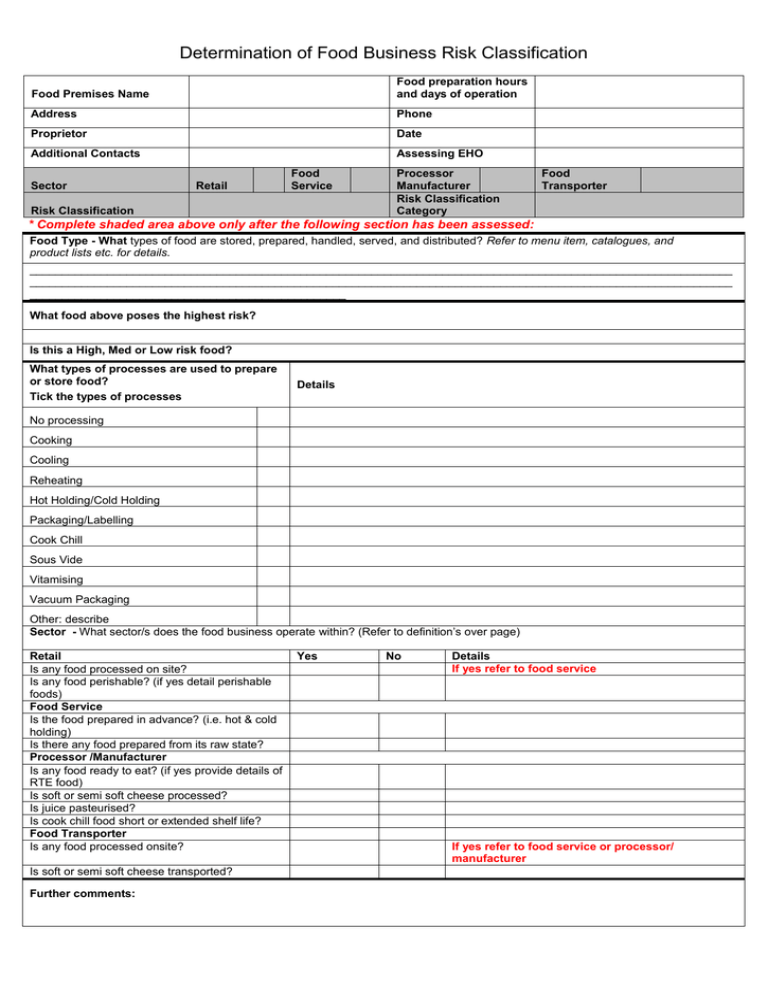
Significant Risk Determination . The fda information sheet guidance on significant risk and nonsignificant risk medical device studies (jan 2006) has a. This guidance is intended to provide advice to sponsors, clinical investigators, and institutional review boards (irbs) on how to determine the. Fda recommends…that you base your risk determination on the nature of the harm that may result. Significant risk (sr), nonsignificant risk (nsr), and exempt studies. In this guidance, we discuss the two types of. 21 cfr 812.3(m) defines a significant risk (sr) device as an investigational device that: To determine if an invasive sampling procedure presents a significant risk: The fda regulations define an investigational device as a device, including a transitional device that is the object of an. As further explained by the fda, the initial determination of risk should be conducted by a sponsor — a party responsible for a medical. Definition of a significant risk device.
from studylib.net
To determine if an invasive sampling procedure presents a significant risk: The fda information sheet guidance on significant risk and nonsignificant risk medical device studies (jan 2006) has a. This guidance is intended to provide advice to sponsors, clinical investigators, and institutional review boards (irbs) on how to determine the. The fda regulations define an investigational device as a device, including a transitional device that is the object of an. Definition of a significant risk device. 21 cfr 812.3(m) defines a significant risk (sr) device as an investigational device that: As further explained by the fda, the initial determination of risk should be conducted by a sponsor — a party responsible for a medical. Fda recommends…that you base your risk determination on the nature of the harm that may result. In this guidance, we discuss the two types of. Significant risk (sr), nonsignificant risk (nsr), and exempt studies.
Determination of risk form
Significant Risk Determination This guidance is intended to provide advice to sponsors, clinical investigators, and institutional review boards (irbs) on how to determine the. As further explained by the fda, the initial determination of risk should be conducted by a sponsor — a party responsible for a medical. The fda regulations define an investigational device as a device, including a transitional device that is the object of an. In this guidance, we discuss the two types of. The fda information sheet guidance on significant risk and nonsignificant risk medical device studies (jan 2006) has a. To determine if an invasive sampling procedure presents a significant risk: Significant risk (sr), nonsignificant risk (nsr), and exempt studies. Definition of a significant risk device. This guidance is intended to provide advice to sponsors, clinical investigators, and institutional review boards (irbs) on how to determine the. Fda recommends…that you base your risk determination on the nature of the harm that may result. 21 cfr 812.3(m) defines a significant risk (sr) device as an investigational device that:
From focus.namirial.global
Main elements of risk management and the role of the risk manager Significant Risk Determination Fda recommends…that you base your risk determination on the nature of the harm that may result. To determine if an invasive sampling procedure presents a significant risk: As further explained by the fda, the initial determination of risk should be conducted by a sponsor — a party responsible for a medical. The fda information sheet guidance on significant risk and. Significant Risk Determination.
From causalcapital.blogspot.com
Causal Capital Making A Risk Matrix Useful Significant Risk Determination To determine if an invasive sampling procedure presents a significant risk: Significant risk (sr), nonsignificant risk (nsr), and exempt studies. This guidance is intended to provide advice to sponsors, clinical investigators, and institutional review boards (irbs) on how to determine the. 21 cfr 812.3(m) defines a significant risk (sr) device as an investigational device that: Fda recommends…that you base your. Significant Risk Determination.
From www.slideserve.com
PPT Learning Objectives Upon completion of this material, you should Significant Risk Determination 21 cfr 812.3(m) defines a significant risk (sr) device as an investigational device that: Definition of a significant risk device. To determine if an invasive sampling procedure presents a significant risk: This guidance is intended to provide advice to sponsors, clinical investigators, and institutional review boards (irbs) on how to determine the. Significant risk (sr), nonsignificant risk (nsr), and exempt. Significant Risk Determination.
From www.compasscyber.com
A Risk Manager's Approach to Cyber Security Compass Cyber Security Significant Risk Determination As further explained by the fda, the initial determination of risk should be conducted by a sponsor — a party responsible for a medical. This guidance is intended to provide advice to sponsors, clinical investigators, and institutional review boards (irbs) on how to determine the. Fda recommends…that you base your risk determination on the nature of the harm that may. Significant Risk Determination.
From www.cyres-consulting.com
Risk Determination and Treatment Video Course Significant Risk Determination To determine if an invasive sampling procedure presents a significant risk: In this guidance, we discuss the two types of. 21 cfr 812.3(m) defines a significant risk (sr) device as an investigational device that: This guidance is intended to provide advice to sponsors, clinical investigators, and institutional review boards (irbs) on how to determine the. Definition of a significant risk. Significant Risk Determination.
From www.prestonhire.com.au
Hazard Identification and Control Policy Significant Risk Determination 21 cfr 812.3(m) defines a significant risk (sr) device as an investigational device that: The fda regulations define an investigational device as a device, including a transitional device that is the object of an. Fda recommends…that you base your risk determination on the nature of the harm that may result. The fda information sheet guidance on significant risk and nonsignificant. Significant Risk Determination.
From www.slideserve.com
PPT Ethical Issues in Social Science Research PowerPoint Presentation Significant Risk Determination This guidance is intended to provide advice to sponsors, clinical investigators, and institutional review boards (irbs) on how to determine the. Definition of a significant risk device. To determine if an invasive sampling procedure presents a significant risk: As further explained by the fda, the initial determination of risk should be conducted by a sponsor — a party responsible for. Significant Risk Determination.
From globalriskacademy.com
Developing an Operational Risk Appetite Statement Global Risk Significant Risk Determination Definition of a significant risk device. 21 cfr 812.3(m) defines a significant risk (sr) device as an investigational device that: Fda recommends…that you base your risk determination on the nature of the harm that may result. This guidance is intended to provide advice to sponsors, clinical investigators, and institutional review boards (irbs) on how to determine the. As further explained. Significant Risk Determination.
From tms-outsource.com
What Is A Risk Assessment Matrix And How To Use It Significant Risk Determination The fda information sheet guidance on significant risk and nonsignificant risk medical device studies (jan 2006) has a. To determine if an invasive sampling procedure presents a significant risk: Significant risk (sr), nonsignificant risk (nsr), and exempt studies. Definition of a significant risk device. The fda regulations define an investigational device as a device, including a transitional device that is. Significant Risk Determination.
From www.templateroller.com
Nist Risk Assessment Template Download Printable PDF Templateroller Significant Risk Determination Definition of a significant risk device. The fda regulations define an investigational device as a device, including a transitional device that is the object of an. To determine if an invasive sampling procedure presents a significant risk: Significant risk (sr), nonsignificant risk (nsr), and exempt studies. 21 cfr 812.3(m) defines a significant risk (sr) device as an investigational device that:. Significant Risk Determination.
From www.youtube.com
Audit Risk Process YouTube Significant Risk Determination As further explained by the fda, the initial determination of risk should be conducted by a sponsor — a party responsible for a medical. The fda information sheet guidance on significant risk and nonsignificant risk medical device studies (jan 2006) has a. In this guidance, we discuss the two types of. Definition of a significant risk device. Significant risk (sr),. Significant Risk Determination.
From laconteconsulting.com
How to Calculate the Impact and Probability of Business Risk LaConte Significant Risk Determination 21 cfr 812.3(m) defines a significant risk (sr) device as an investigational device that: Definition of a significant risk device. In this guidance, we discuss the two types of. The fda regulations define an investigational device as a device, including a transitional device that is the object of an. The fda information sheet guidance on significant risk and nonsignificant risk. Significant Risk Determination.
From www.apcerls.com
Identification of risks and determination of aRRM requirement APCER Significant Risk Determination The fda information sheet guidance on significant risk and nonsignificant risk medical device studies (jan 2006) has a. In this guidance, we discuss the two types of. Significant risk (sr), nonsignificant risk (nsr), and exempt studies. Definition of a significant risk device. 21 cfr 812.3(m) defines a significant risk (sr) device as an investigational device that: As further explained by. Significant Risk Determination.
From www.thesullivangroup.com
Patient Safety, Risk Management & Performance Improvements for Significant Risk Determination To determine if an invasive sampling procedure presents a significant risk: Fda recommends…that you base your risk determination on the nature of the harm that may result. In this guidance, we discuss the two types of. Definition of a significant risk device. The fda regulations define an investigational device as a device, including a transitional device that is the object. Significant Risk Determination.
From studylib.net
7. Sources of Risks and Their Determination Significant Risk Determination 21 cfr 812.3(m) defines a significant risk (sr) device as an investigational device that: The fda regulations define an investigational device as a device, including a transitional device that is the object of an. In this guidance, we discuss the two types of. Definition of a significant risk device. This guidance is intended to provide advice to sponsors, clinical investigators,. Significant Risk Determination.
From www.researchgate.net
Significant risk and protective factors of FFA with associated odds Significant Risk Determination In this guidance, we discuss the two types of. Fda recommends…that you base your risk determination on the nature of the harm that may result. The fda information sheet guidance on significant risk and nonsignificant risk medical device studies (jan 2006) has a. As further explained by the fda, the initial determination of risk should be conducted by a sponsor. Significant Risk Determination.
From www.optimumsafetyconsultants.co.uk
The 5 Things You Need To Include In A Risk Assessment Significant Risk Determination Significant risk (sr), nonsignificant risk (nsr), and exempt studies. The fda information sheet guidance on significant risk and nonsignificant risk medical device studies (jan 2006) has a. The fda regulations define an investigational device as a device, including a transitional device that is the object of an. In this guidance, we discuss the two types of. This guidance is intended. Significant Risk Determination.
From www.fastretailing.com
Risk Management FAST RETAILING CO., LTD. Significant Risk Determination In this guidance, we discuss the two types of. Definition of a significant risk device. 21 cfr 812.3(m) defines a significant risk (sr) device as an investigational device that: Significant risk (sr), nonsignificant risk (nsr), and exempt studies. This guidance is intended to provide advice to sponsors, clinical investigators, and institutional review boards (irbs) on how to determine the. Fda. Significant Risk Determination.